Chapter 52 Reading Guide Biotic and Abiotic Factors
Abstract
Biotic and abiotic factors are increasingly acknowledged to synergistically shape broad-scale species distributions. However, the relative importance of biotic and abiotic factors in predicting species distributions is unclear. In particular, biotic factors, such as predation and vegetation, including those resulting from anthropogenic land-use change, are underrepresented in species distribution modeling, but could improve model predictions. Using generalized linear models and model selection techniques, we used 129 estimates of population density of wild pigs (Sus scrofa) from 5 continents to evaluate the relative importance, magnitude, and direction of biotic and abiotic factors in predicting population density of an invasive large mammal with a global distribution. Incorporating diverse biotic factors, including agriculture, vegetation cover, and large carnivore richness, into species distribution modeling substantially improved model fit and predictions. Abiotic factors, including precipitation and potential evapotranspiration, were also important predictors. The predictive map of population density revealed wide-ranging potential for an invasive large mammal to expand its distribution globally. This information can be used to proactively create conservation/management plans to control future invasions. Our study demonstrates that the ongoing paradigm shift, which recognizes that both biotic and abiotic factors shape species distributions across broad scales, can be advanced by incorporating diverse biotic factors.
Introduction
Predicting and mapping species distributions, including geographic range and variability in abundance, is fundamental to the conservation and management of biodiversity and landscapes1. The ecological niche defines species-habitat relationships2,3,4 and provides a useful framework for understanding the range and abundance of species in relation to biotic and abiotic factors. Further, niche relationships across local scales can provide novel information about the ecology, conservation, and management of species at macro scales5. Most studies evaluating a species' niche across their distribution focus on presence-absence occurrence data to predict the geographic range6; however, conservation and management plans for species can be improved by understanding patterns of population abundance and density across a species' range7. In particular, evaluating population density, compared to occurrence, can reveal novel patterns of species distributions in relation to landscape factors8.
There is an ongoing paradigm shift in understanding how biotic and abiotic factors shape species distributions. Until recently, it was widely accepted that abiotic factors, such as temperature and precipitation, played the primary role in shaping distributions of species and biodiversity at broad scales (e.g., regional, continental, global extents) and that biotic factors were most important at fine scales (e.g., site, local extents)9,10,11. It is increasingly recognized, however, that biotic factors are important determinants of species distributions at broad spatial scales, especially when considering biotic interactions12,13,14,15,16. Although interspecific competition can be an important biotic determinant in species distribution models at broad scales, other forms of biotic interactions, such as predation and symbioses, can also be important determinants15,17, but have received less attention18. In addition, although researchers have evaluated the effects of biotic interactions on geographic range limits18, relatively few studies have evaluated how biotic factors influence population density across a species' range19,20, which can be more informative in understanding macro-ecological patterns7,21.
In addition to species interactions, biotic factors related to vegetation can influence species distributions and abundance at broad scales. In particular, anthropogenic land-use change is rarely considered when evaluating species distributions at broad scales; however, given the human footprint globally22 and projections for expanding human impacts on the environment23,24, biotic factors created by human activities are potentially important predictors that can contribute to a better understanding of species distributions8. For example, agricultural crops are a dominant biotic factor across continents that are facilitated by human engineering and the redistribution of ecological resources and energy, which can have profound impacts on plant and animal populations across broad extents; agriculture can increase populations for some species through increased food, resource availability, and landscape heterogeneity, or decrease populations due to loss of habitat25,26,27. Ultimately, further evaluation is necessary to understand the relative importance of abiotic and biotic factors in shaping species distributions across broad spatial scales13,15.
Invasive species are a primary driver of widespread and severe negative impacts to ecosystems, agriculture, and humans across local to global scales28. These introduced plants and animals often exhibit broad geographic distributions, can be relatively well studied across local scales, and provide novel opportunities to evaluate broad-scale patterns of niche relationships29. Predictions of potential geographic distribution of invasive species can provide critical information that can inform the prevention, eradication, and control of populations, which has been evaluated for many taxa, including plants30, amphibians31, and invertebrates32. However, few studies have predicted the potential ranges and abundance of non-native mammals33. Especially for wide-ranging species that can occur across broad extents of landscapes, predictions of how population density varies spatially can provide important information for prioritizing conservation and management actions.
Few species exhibit a global distribution that extends across Europe, Asia, Africa, North and South America, Australia, and oceanic Islands. Besides naturalized animals, such as the house mouse (Mus musculus) and brown rat (Rattus norvegicus), wild pigs (Sus scrofa; other common names include wild boar, wild/feral swine, wild/feral hog, and feral pig) have one of the widest geographic distributions of any mammal; further, it exhibits the widest geographic range of any large mammal34, with the exception of humans. The expansive global distribution of wild pigs is attributed to its broad native range in Eurasia and northern Africa, widespread introduction by humans outside its native range, and superior adaptability, where it occurs in a wide variety of ecological communities, ranging from deserts to temperate and tropical environments35,36, with a corresponding diverse omnivorous diet37. Across its non-native range (Fig. 1; Supplementary Methods S1), including North and South America, Australia, sub-Saharan Africa, and many islands, wild pigs are considered one of the 100 most harmful invasive species in the world38 due to wide-ranging ecosystem disturbance, agricultural damage, pathogen and disease vectors to wildlife, livestock and people, and social impacts to people and property39,40,41. Wild pigs are therefore a model species to evaluate biotic and abiotic factors associated with population density because they exhibit a global distribution across six continents, are widely studied across much of their native and non-native (i.e., invasive or introduced) ranges, and previous research has indicated that their population density was related to abiotic factors across a continental scale, although it was ambiguous how biotic factors shape their abundance, warranting further study42.

Areas of white indicate locations in which wild pigs are likely not present. This map was created using ArcGIS 10.3.198. See Supplementary Methods S1 for a description of methods and citations used for creating the map of wild pig global distribution across its native and non-native ranges.
Full size image
To address these ecological questions and understand the relative importance of biotic and abiotic factors in shaping the global distribution of a highly invasive mammal, we evaluated estimates of population density of wild pigs across diverse environments on five continents. Specifically, we (1) evaluate how biotic (i.e., vegetation and predation) and abiotic (i.e., climate) factors (Table 1) shape population density across a global scale and (2) create a predictive distribution map of potential population density across the world. We also compare population density between island and mainland populations. Our results contribute novel insight into the relative roles of biotic and abiotic factors in shaping the distribution of species' population densities across continental and global scales, particularly relating to human-mediated land-use change, which can provide critical information to management and conservation strategies.
Full size table
Results
We compiled 147 estimates of wild pig density (# animals/km2), which resulted in 129 estimates of density across their global distribution used in our analyses (Fig. 1; Supplementary Table S2). Some areas contained > 1 density estimate, and these were averaged. Population density of wild pigs was higher on islands (n = 11) compared to on the mainland (n = 118) (t = 4.72, df = 10.93, p < 0.001; Supplementary Figure S3). For the untransformed density estimates, mean population density for on the mainland equaled 2.75 (se = 0.38) and islands equaled 18.52 (se = 4.15). The highest estimates of population density occurred on islands, which reached upwards of 40 wild pigs/km2 (Supplementary Table S2). Due to differences in population density between islands and on the mainland, we used density estimates from mainland populations in our subsequent analyses.
Population density was influenced by both biotic and abiotic factors across the global distribution (Tables 2 and 3; Supplementary Table S4). The suite of best models all included combinations of biotic and abiotic factors (Table 2) and the top model (AICc = 237.94; model weight = 0.68; adjusted R2 = 0.55) had > 1,000 times more support as the best approximating model than the top model considering only abiotic factors (AICc = 311.30; model weight = 7.94 × 10−17) (Supplementary Table S4). The variables with the greatest importance included potential evapotranspiration, large carnivore richness, precipitation during the wet and dry seasons, unvegetated area, and agriculture, which also exhibited 95% confidence intervals that did not overlap zero (Table 3). Density was greatest at moderate levels of potential evapotranspiration and agriculture, decreased with large carnivore richness and amount of unvegetated area, and increased with precipitation during the wet and dry seasons (Fig. 2); percent forest cover was unsupported in models when considering the suite of variables in analyses.
Full size table
Full size table
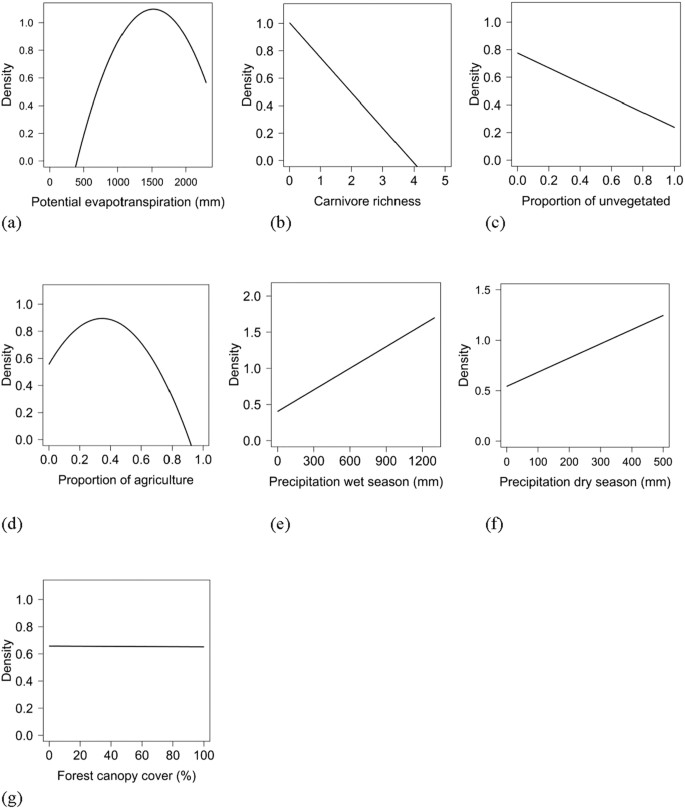
Relationships of biotic and abiotic factors with population density (natural log scale; #/km2) of wild pigs, including potential evapotranspiration (a), large carnivore richness (b), unvegetated (c), agriculture (d), precipitation during the wettest season (e), precipitation during the driest season (f), and forest canopy cover (g).
Full size image
Using the full model-averaged results of parameter estimates, we created a predictive map of global wild pig population density (Fig. 3; Supplementary Figure S5). Wild pig populations were predicted to occur at low to high population densities across all continents, including large areas of land where wild pigs are currently absent. The highest predicted densities occurred in southeastern, eastern, and western North America, throughout Central America, northern, eastern, and southwestern South America, western, southern, and eastern Eurasia, throughout Indonesia, central and southern Africa, and northern and southeastern Australia (Fig. 3; Supplementary Figure S5). Results of k-fold cross validation demonstrated that the model had good predictive ability with a mean squared prediction error (MSPE) of 0.22 and a Pearson's correlation between observed and predicted values of 0.80 (t = 17.711, df = 181, p-value < 0.001).
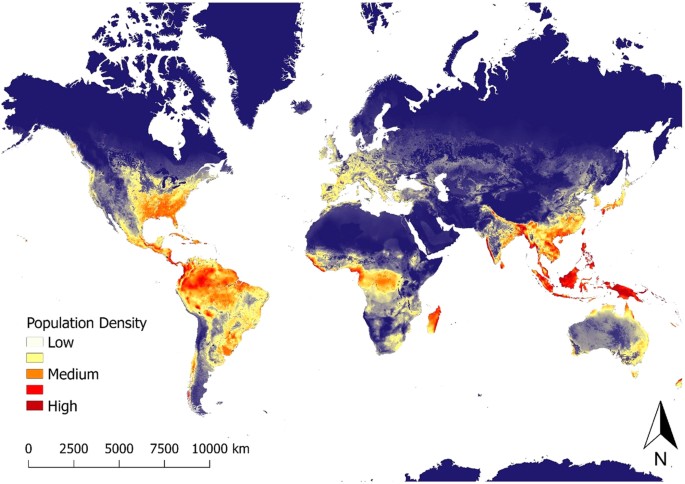
For terrestrial environments, areas of white represent low density (1 individual/km2), orange moderate density (6 individuals/km2), and dark red high density (≥11 individuals/km2). Maps were created using Google Earth Engine80 and QGIS 2.14.390. See Supplementary Figure S5 for finer scale maps of predicted population density of wild pigs for Europe, Asia, Africa, Australia, North America, and South America.
Full size image
Discussion
Population density of an invasive large mammal was strongly influenced by both biotic and abiotic factors across its global distribution. Consistent with the prediction that abiotic factors drive broad-scale patterns of species distribution, potential evapotranspiration (PET) and precipitation variables were important predictors of population density on a global scale. In addition, contributing to growing evidence that biotic factors are also important determinants of broad-scale patterns of species distributions, both biotic interactions and vegetation played important roles in predicting the distribution of wild pig populations globally. Further, land-use change mediated by human activities strongly predicted the broad-scale distribution of an invasive large mammal. Consistent with previous studies evaluating how population density of ungulates varied across broad scales, both bottom-up (resource-related) and top-down (predation) factors influenced the distribution of wild pig populations19,42,43. Ultimately, wild pig populations across their global distribution appeared to respond to biotic and abiotic factors related to plant productivity, forage and water availability, cover, predation, and anthropogenic land-use change.
Using both biotic and abiotic factors to evaluate broad-scale species distributions can create more realistic maps of range and density with better predictive ability16,44, which can better inform management and conservation strategies for species. For example, population density of wild pigs was highest in landscapes with moderate levels of agriculture and PET, lower large carnivore richness and amount of unvegetated area, and greater precipitation during the wet and dry seasons. Using these relationships, we created a predictive map of population density across the world, which can be used to manage existing populations and predict areas where wild pig populations are likely to expand or invade if given the opportunity. Ultimately, this information can be used to prioritize management activities in areas at risk of invasion and with expanding populations.
Abiotic factors, such as temperature and precipitation, are consistently found to be primary determinants of species distributions at broad scales11. Potential evapotranspiration can be especially informative for understanding broad-scale ecological patterns45, such as species distributions. This was supported in our research where PET was the most important predictor of population density across the global distribution of wild pigs. Potential evapotranspiration is highly correlated with temperature variables, thus indicating that wild pig density was greatest at relatively moderate temperatures and density was lower in areas exhibiting extreme low and high temperatures. In addition, the strong support of precipitation variables in our models is consistent with the association of wild pigs with vegetation cover, forage, and water36. In particular, precipitation likely facilitates rooting behavior by wild pigs by softening the soil substrate46.
Biotic factors were among the most supported variables predicting population density across a global scale. Our results indicated that the presence of large carnivores can influence wild pig population density. Large carnivore richness was strongly supported in our models and exhibited a negative relationship with wild pig density; as the number of large carnivore species increased, wild pig density decreased, which is consistent with studies in Eurasia and Australia42,47,48. In addition, interspecific competition can influence the distribution of species and it has been hypothesized that wild pigs have not extensively invaded wildlands in some regions of sub-Saharan Africa due to the presence of other pig species that exhibit similar niches49. Although competition with other species might influence wild pig populations and their distribution49,50,51, in other cases wild pigs are reported to spatially and temporally partition habitat use to reduce niche overlap with potential competitors52,53,54 and not show evidence for interference competition with related mammals (e.g., species within the suborder Suiformes), such as native peccary species55, thus, it is unclear how interspecific interactions influence wild pig populations across their global distribution. Further, understanding potential interspecific competition for invasive species can be especially challenging in non-native habitat because invaders have not coevolved with competitors or predators and thus it is difficult to predict which species will be subordinate or dominant in potential competitive interactions or how competition might influence species distributions in unoccupied habitat17,18,56. Because it was unknown how competitive interactions between wild pigs and other species might influence their distribution, particularly outside their native range, competition was not included in our analyses. To understand how competition between non-native and native species influences species distributions, field studies evaluating interspecific competition are necessary across the wild pig's native and non-native geographic range, particularly across local spatial scales.
Although biotic interactions between animals are the primary biotic factors evaluated in species distribution models at broad scales, the role of plant communities has received less consideration. In particular, anthropogenic land-use change increasingly influences vegetation communities across continents and warrants a better understanding for how human activities are shaping broad-scale distributions of plant and animal populations22,24. For example, agriculture is a dominating land cover type across continents23,25, which can potentially benefit species distributions in at least two ways. Agriculture can (1) increase population density within areas of a species' current geographic range through supplemental food and increased resource availability and (2) allow geographic ranges to expand by creating habitat in areas that were previously unsuitable. In contrast, as agriculture increasingly dominates landscape patterns at broad extents, cover and other resources correspondingly decrease, which can negatively impact the geographic range and population density of some species. Our results demonstrate that agriculture can produce both positive and negative effects on populations, depending on the levels of agriculture. At intermediate levels of agriculture, population density of wild pigs was greatest, likely due to an optimal mix of food and cover. Whereas, at high levels of agriculture, population density decreased precipitously, which was likely a result of inadequate cover. Our results indicate that heterogeneous landscapes with a mix of agriculture and cover will support the greatest populations of wild pigs, which is consistent with broad-scale patterns of wild pig populations in North America and Eurasia57,58,59. Due to relatively high predicted population densities of wild pigs inhabiting heterogeneous landscapes, these regions would likely experience the greatest crop damage, leading to high economic loss to farmers.
Forest is considered a key habitat type preferred by wild pigs59,60. In univariate analyses, forest was an important positive predictor of wild pig density (β = 0.170, se = 0.056). When considering additional predictor variables in our models, however, forest was relatively unimportant in predicting wild pig density, which is also consistent when evaluating wild pig occurrence over broad scales57. Thus, the interpretation of how forest influences the distribution of wild pigs must be considered in the context of other variables included in models, where abiotic factors might adequately explain forest distribution (see discussion below). However, as predicted, vegetation and cover play a strong role in predicting wild pig density; as the amount of unvegetated area increased across the landscape, wild pig population density decreased, which is consistent with geographic distribution maps of wild pigs61.
In some systems, abiotic factors can be stronger predictors of species distributions, than biotic factors, because of high correlations between these two factors62. Our study indicated that both factors can be important predictors of species distributions, potentially because abiotic factors may poorly predict biotic factors stemming from human activities. In addition, human influences might weaken the correlation between abiotic and biotic factors. For example, humans can significantly reduce the number of large carnivores in an area63, although these species would be predicted to occur across broad areas based on abiotic factors and historic biotic conditions. In addition, human land use change can lead to unpredictable biotic patterns in relation to abiotic factors, such as through agricultural landscape conversion. Although soil types might support crop production, many agricultural areas occur in arid landscapes requiring irrigation of water and application of fertilizer to maintain production25. Thus, agricultural crops could not grow in many areas based on broad-scale climate factors alone, and therefore, abiotic factors can be poor predictors of agricultural practices in some regions. Indeed, there likely are other examples where abiotic and biotic factors may exhibit low correlation in some systems (e.g., location of human activities and development, altered interspecific interactions due to human activities, and other forms of anthropogenic land use change). Ultimately, it can be useful to consider biotic factors in species distribution models that might be poorly predicted by abiotic factors due to human activities.
Additional biotic factors that can influences species distributions on a broad scale, particularly invasive species, include the role of humans in distributing the founding individuals of new populations. For example, invasive wild pig populations have arisen across several continents recently through human activities. Illegal translocations by humans for hunting purposes can facilitate the long-distance expansion of wild pig populations into new areas64,65,66, which is currently a primary source of new populations globally39,41. Further, in countries such as Canada, Brazil, and Sweden, wild pig farms were the propagule source for recent populations of wild pigs across broad regions, which are currently spreading into new areas67,68,69. Indeed, propagule pressure (i.e., the number of individuals introduced and release events) determines both the likelihood of invasive species becoming established, as well as the rate of geographic range expansion60,70. In addition, invasive species that exhibit r-selected characteristics (e.g., early maturity, short generation time, and high fecundity) can be more likely to successfully invade novel landscapes71. Even at low population densities, invasive species with high reproductive output are more likely to establish populations in areas of lower quality habitat72. Given that wild pigs are one of the most fecund large mammals (e.g., mean litter sizes ranging from 3.0 to 8.4 piglets per sow with the potential for >1 litter annually)36, their reproductive characteristics might increase the probability of establishment and enable them to compensate for small population sizes when introduced into novel environments across a range of habitat qualities.
Population density, compared to presence-absence occurrence, can provide more informative conclusions of species distributions in relation to biotic and abiotic factors7,8. For example, although large carnivores likely do not exclude wild pigs from habitat across broad scales, our study revealed they can influence abundance. However, occurrence of species would remain constant across varying population densities, unless it resulted in species exclusion. Ultimately, population densities can provide more detailed information about species distributions, which can better inform conservation and management plans and policy7. Studies analyzing presence-only data with logistic regression and Maximum Entropy (MaxEnt) models have examined methods to address spatial sampling bias73,74,75 and additional evaluations would be useful for studies using population density data with multiple linear regression. Further, global analyses of population genetics could be used to identify groups and the proportion of wild and domestic genes across wild pig populations, which could be used to incorporate population structure into analyses to better understand population characteristics.
Predicting species distributions provides critical information to the management and conservation of biodiversity, especially for controlling invasive species. Without intensive management actions, our study predicts that there is strong potential for wild pigs to expand their geographic range and further invade expansive areas of North America, South America, Africa, and Australia. Although wild pigs currently occupy broad regions of predicted habitat in their non-native range, many regions of predicted habitat are currently unoccupied and may be at high risk for future invasion. These areas might warrant increased surveillance by local, state, and federal agencies to counter the establishment of populations. Although attention in unoccupied areas that are predicted to support high densities of wild pigs might warrant priority for countering population introductions, wild pigs can persist in relatively low quality habitat (e.g., arid and/or cold regions) and these areas also warrant attention to halt invasions. Given the potential for wild pig populations to rapidly expand once established36, predictions of potential population density in unoccupied habitat can provide critical information to land managers, which can be used to proactively develop management plans to prevent introductions and control or eradicate populations if they become introduced.
Methods
Density Estimates
To evaluate the population density (i.e., number of individuals per unit area) of wild pigs throughout their global distribution, we compiled density estimates from the literature throughout its native and non-native ranges across each continent and island for which data were available (Supplementary Table S1). Previous research evaluated how population density of wild pigs varied across western Eurasia42 and we incorporated these 54 estimates of population density into our analysis. In addition, we followed the methodological recommendation of Melis et al.42. to average data when multiple estimates were available for >1 season or year at a study area. Island populations typically exhibit higher population density compared to mainland populations76,77. We thus compared estimates of wild pig population density between island and mainland populations; if population density for islands was significantly higher than on the mainland, we focused on only evaluating mainland populations in subsequent analyses.
Models evaluating and predicting species distributions can be improved by including areas of absence (a.k.a., pseudo-absence or background locations) or zero density to sample the full range of available landscape conditions1 to predict the potential range of a species, absence locations should occur outside the environmental domain of the species, but within a reasonable distance of the species' geographic range78. Because wild pigs have occurred within their native range for thousands of years, we assumed that populations were at equilibrium and the species had colonized available habitat associated with its geographic distribution. Thus, regions adjacent to its native distribution that were classified as unoccupied were assumed to be unsuitable for population persistence due to unfavorable environmental conditions. In addition, spatial sampling bias (i.e., uneven sampling across geographic extents) can be addressed by increasing the number of background locations in areas with greater sampling73,74. The majority of density estimates used in our study occurred within the wild pig's native range of Europe and Asia and we focused sampling of background locations associated with this region. To include locations with estimates of zero density in our analyses, we used a three-step approach. First, we created a buffered region that occurred across the area between 100–1000 km around the boundary of the wild pig's native range79. Next, we calculated the spatial extent of the native range and buffered regions. Lastly, accounting for the area of each region, we selected a random sample of locations within the buffered region that was proportional to the number of estimates used in the native terrestrial range of wild pigs. Based on this approach, we used 65 locations of zero density in our analyses that occurred across central Russia, Mongolia, western China, Saudi Arabia, and northern African countries. Zero density estimates were used in analyses relating wild pig density to landscape variables and excluded when comparing population density between island and mainland populations.
Landscape Variables
We considered a suite of biotic and abiotic landscape variables, which were divided into vegetation, predation, and climate factors (Table 1) that we hypothesized to influence population density of wild pigs. We used landscape variables that were available globally and, where possible, over long time periods (i.e., estimates averaged over several decades) that coincide with the density estimates we compiled for our analyses. Geospatial data layers were acquired through either Google Earth Engine80 or were downloaded from online sources (Table 1).
The biotic factors that we evaluated included agriculture, broadleaf forest, enhanced vegetation index (EVI), forest canopy cover, difference in the proportion between forest and agriculture (to characterize landscape heterogeneity), normalized difference vegetation index (NDVI), large carnivore richness, and unvegetated area (Table 1). We expected a positive relationship between density and all vegetation factors, except unvegetated area, due to their association with increased food availability, plant productivity, and cover. In addition, we expected a quadratic relationship between population density and agriculture because we predicted density to be greatest at moderate levels of agriculture (due to a mix of cover and food) and low at high levels of agriculture (due to a lack of adequate cover). Finally, we expected a negative relationship between population density and large carnivore richness.
The abiotic factors that we evaluated included two measures of ecological energy regimes, actual evapotranspiration (the amount of water loss from evaporation and transpiration, which is related to plant productivity) and potential evapotranspiration (PET; the amount of evaporation and transpiration that would occur with a sufficient water supply, considering solar radiation, air temperature, humidity, and wind speed;45). Actual evapotranspiration is a measure of water-energy balance and potential evapotranspiration is considered a measure of ambient energy and often highly correlated with temperature variables81. Although evapotranspiration variables can include elements of biotic (i.e., transpiration from plants) and abiotic (i.e., climate and water) factors, they were classified as abiotic for our analyses. In addition, we evaluated precipitation during dry and wet seasons, and annually, and temperature during summer and winter, and annually (Table 1). We predicted a positive relationship between density and precipitation variables due to associated increases in forage, water, and cover and quadratic relationships between density and evapotranspiration and temperature variables due to expected peak densities at intermediate levels and low densities at low and high levels.
Modeling
We used data from the wild pig's native and non-native range in our modeling. Although niche shifts between a species' native and non-native range appear to be uncommon and it is often assumed that species exhibit niche stasis or conservatism30,82,83,84 through space and time, models that use data only from a species' native range can exhibit poor predictive power in the species' non-native range85,86,87. Therefore, it is important to include data from the species' entire distribution to increase the predictive ability of models across both the native and non-native ranges32,88,89. Because wild pigs have been established across much of their non-native range for an extended period of time (e.g., typically greater than a century), we assumed that populations used in our analyses had achieved a localized equilibrium with their environment.
All geospatial data layers were evaluated using QGIS90 and Google Earth Engine80 and statistical analyses were conducted using R91. Because there is uncertainty about the exact location of studies and the scale in which processes might influence wild pig densities, we evaluated multiple scales for each covariate using 10, 20, and 40 km radius buffers around the location of each density estimate (Table 1). Thus a moving window approach was conducted so that each pixel within a spatial layer summarized the landscape within the buffered radius. To determine the best scale for analyses we used a multi-criteria approach. First, variables were centered and scaled to improve model fit92. Next, we considered quadratic relationships for landscape factors that were predicted to exhibit a curvilinear pattern (Table 1). Last, we selected the best scale and relationship for each covariate based on wild pig ecology, model comparisons using Akaike's Information Criterion corrected for small sample size AICc;93, and plots of residuals. Once the appropriate scale was determined for each variable (Table 1), we evaluated the Pearson correlation among all variables and excluded highly correlated variables (r > 0.70) from our final analysis.
We used multiple linear regression to evaluate how population density was influenced by our final suite of biotic and abiotic factors (Table 1). The distribution of density estimates were right skewed, thus we log-transformed density estimates using the natural logarithm42. To compare the relative importance of biotic and abiotic factors and to determine parameter estimates of variables, we ranked all possible models using AICc, model-averaged parameter estimates (i.e., full conditional), and calculated variable importance values93,94,95. We used model weights and evidence ratios to evaluate if biotic factors improved model fit by comparing models including only abiotic factors to models also including biotic factors. Model averaged parameter estimates were used to create a predictive global map of wild pig density (1 km2 resolution). This map displays the maximal potential density of wild pigs in relation to the biotic and abiotic factors used in our modeling and reflects predicted densities that would be achieved if wild pigs had access to all landscapes, their movements were unrestricted, and management activities did not suppress populations. We validated our model using mean squared prediction error (MSPE)96 and k-fold cross validation and selected the number of bins based on Huberty's rule of thumb (k = 4)97.
Additional Information
How to cite this article: Lewis, J. S. et al. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 7, 44152; doi: 10.1038/srep44152 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1
Franklin, J. Mapping species distributions: spatial inference and prediction. (Cambridge University Press, 2009).
- 2
Grinnell, J. The niche-relationships of the California Thrasher. The Auk 34, 427–433 (1917).
Google Scholar
- 3
MacArthur, R. H. In Population Biology and Evolution(ed R. C. Lewontin ) 159–186 (Syracuse University Press, 1968).
- 4
Hutchinson, G. E. Concluding remarks. Cold Spring Harbor Symposium on Quantitative Biology 22, 415–427 (1957).
Google Scholar
- 5
Brown, J. H. Macroecology: progress and prospect. Oikos 87, 3–14 (1999).
Google Scholar
- 6
Elith, J. & Leathwick, J. R. Species distribution models: ecological explanation and prediction across space and time. Annual Review of Ecology, Evolution, and Systematics 40, 677–697 (2009).
Google Scholar
- 7
Brown, J. H., Mehlman, D. W. & Stevens, G. C. Spatial variation in abundance. Ecology 76, 2028–2043 (1995).
Google Scholar
- 8
Randin, C. F., Jaccard, H., Vittoz, P., Yoccoz, N. G. & Guisan, A. Land use improves spatial predictions of mountain plant abundance but not presence-absence. Journal of Vegetation Science 20, 996–1008 (2009).
Google Scholar
- 9
Pearson, R. G. & Dawson, T. P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography 12, 361–371 (2003).
Google Scholar
- 10
Benton, M. J. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732 (2009).
ADS CAS Google Scholar
- 11
Wiens, J. J. The niche, biogeography and species interactions. Philosophical Transactions of the Royal Society of London B: Biological Sciences 366, 2336–2350 (2011).
Google Scholar
- 12
Van der Putten, W. H., Macel, M. & Visser, M. E. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2025–2034 (2010).
Google Scholar
- 13
Meier, E. S. et al. Biotic and abiotic variables show little redundancy in explaining tree species distributions. Ecography 33, 1038–1048 (2010).
Google Scholar
- 14
Guisan, A. & Thuiller, W. Predicting species distribution: offering more than simple habitat models. Ecology Letters 8, 993–1009 (2005).
Google Scholar
- 15
Wisz, M. S. et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biological Reviews 88, 15–30 (2013).
Google Scholar
- 16
Leach, K., Montgomery, W. I. & Reid, N. Modelling the influence of biotic factors on species distribution patterns. Ecological Modelling 337, 96–106 (2016).
Google Scholar
- 17
Anderson, R. P. When and how should biotic interactions be considered in models of species niches and distributions? Journal of Biogeography, doi: 10.1111/jbi.12825 (2016).
- 18
Sexton, J. P., McIntyre, P. J., Angert, A. L. & Rice, K. J. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics 40, 415–436 (2009).
Google Scholar
- 19
Melis, C. et al. Predation has a greater impact in less productive environments: variation in roe deer, Capreolus capreolus, population density across Europe. Global Ecology and Biogeography 18, 724–734 (2009).
Google Scholar
- 20
Pasanen-Mortensen, M., Pyykönen, M. & Elmhagen, B. Where lynx prevail, foxes will fail–limitation of a mesopredator in Eurasia. Global Ecology and Biogeography 22, 868–877 (2013).
Google Scholar
- 21
Boulangeat, I., Gravel, D. & Thuiller, W. Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecology Letters 15, 584–593 (2012).
PubMed PubMed Central Google Scholar
- 22
Sanderson, E. W. et al. The Human Footprint and the Last of the Wild. Bioscience 52, 891–904 (2002).
Google Scholar
- 23
Laurance, W. F., Sayer, J. & Cassman, K. G. Agricultural expansion and its impacts on tropical nature. Trends in Ecology & Evolution 29, 107–116 (2014).
Google Scholar
- 24
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
ADS CAS Google Scholar
- 25
Alexandratos, N. & Bruinsma, J. World agriculture towards 2030/2050: the 2012 revision. (ESA Working Paper No. 12-03, Rome, FAO, 2012).
- 26
Green, R. E., Cornell, S. J., Scharlemann, J. P. & Balmford, A. Farming and the fate of wild nature. Science 307, 550–555 (2005).
ADS CAS Google Scholar
- 27
Bengtsson, J., Ahnström, J. & Weibull, A.-C. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. Journal of Applied Ecology 42, 261–269 (2005).
Google Scholar
- 28
Mack, R. N. et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications 10, 689–710 (2000).
Google Scholar
- 29
Parmesan, C. et al. Empirical perspectives on species borders: from traditional biogeography to global change. Oikos 108, 58–75 (2005).
Google Scholar
- 30
Peterson, A. T. Predicting the geography of species' invasions via ecological niche modeling. The Quarterly Review of Biology 78, 419–433 (2003).
Google Scholar
- 31
Ficetola, G. F., Thuiller, W. & Miaud, C. Prediction and validation of the potential global distribution of a problematic alien invasive species—the American bullfrog. Diversity and Distributions 13, 476–485 (2007).
Google Scholar
- 32
Sánchez-Fernández, D., Lobo, J. M. & Hernández-Manrique, O. L. Species distribution models that do not incorporate global data misrepresent potential distributions: a case study using Iberian diving beetles. Diversity and Distributions 17, 163–171 (2011).
Google Scholar
- 33
Kauhala, K. & Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: history of colonization, features behind its success, and threats to native fauna. Current Zoology 57, 584–598 (2011).
Google Scholar
- 34
Oliver, W. L. R. & Brisbin, I. In Pigs, peccaries and Hippos: status survey and conservation action plan(ed W. L. R. Oliver ) 179–195 (IUCN, 1993).
- 35
Oliver, W. L. R., Brisbin, I. L. & Takahashi, S. In Pigs, peccaries and Hippos: status survey and conservation action plan(ed W. L. R. Oliver ) 112–120 (IUCN, 1993).
- 36
Mayer, J. & Brisbin, I. L. Wild pigs: biology, damage, control techniques and management. (Savannah River Site Aiken, SC, USA, 2009).
- 37
Ballari, S. A. & Barrios-García, M. N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mammal Review 44, 124–134 (2014).
Google Scholar
- 38
Lowe, S., Browne, M., Boudjelas, S. & De Poorter, M. 1 00 of the world's worst invasive alien species: A selection from the global invasive species database. 1–12 (Aukland, New Zealand, 2000).
- 39
Barrios-Garcia, M. N. & Ballari, S. A. Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biological Invasions 14, 2283–2300 (2012).
Google Scholar
- 40
Courchamp, F., Chapuis, J.-L. & Pascal, M. Mammal invaders on islands: impact, control and control impact. Biological Reviews 78, 347–383 (2003).
Google Scholar
- 41
Bevins, S. N., Pedersen, K., Lutman, M. W., Gidlewski, T. & Deliberto, T. J. Consequences associated with the recent range expansion of nonnative feral swine. Bioscience 64, 291–299 (2014).
Google Scholar
- 42
Melis, C., Szafrańska, P. A., Jędrzejewska, B. & Bartoń, K. Biogeographical variation in the population density of wild boar (Sus scrofa) in western Eurasia. Journal of Biogeography 33, 803–811 (2006).
Google Scholar
- 43
Danell, K., Bergström, R., Duncan, P. & Pastor, J. Large herbivore ecology, ecosystem dynamics and conservation. Vol. 11 (Cambridge University Press, 2006).
- 44
González-Salazar, C., Stephens, C. R. & Marquet, P. A. Comparing the relative contributions of biotic and abiotic factors as mediators of species' distributions. Ecological Modelling 248, 57–70 (2013).
Google Scholar
- 45
Fisher, J. B., Whittaker, R. J. & Malhi, Y. ET come home: potential evapotranspiration in geographical ecology. Global Ecology and Biogeography 20, 1–18 (2011).
Google Scholar
- 46
Sandom, C. J., Hughes, J. & Macdonald, D. W. Rooting for rewilding: quantifying wild boar's Sus scrofa rooting rate in the Scottish Highlands. Restoration Ecology 21, 329–335 (2013).
Google Scholar
- 47
Woodall, P. F. Distribution and population dynamics of dingoes (Canis familiaris) and feral pigs (Sus scrofa) in Queensland, 1945-1976. Journal of Applied Ecology 20, 85–95 (1983).
Google Scholar
- 48
Ickes, K. Hyper-abundance of native wild pigs (Sus scrofa) in a lowland Dipterocarp rain forest of peninsular Malaysia Biotropica 33, 682–690 (2001).
Google Scholar
- 49
Oliver, W. & Fruzinski, B. In Biology of Suidae(eds R. H. Barrett & F. Spitz ) 93–116 (Institute National de Recherche Agronomique, Castanet, France, 1991).
- 50
Jedrzejewska, B., Jedrzejewski, W., Bunevich, A. N., Milkowski, L. & Krasinski, Z. A. Factors shaping population densities and increase rates of ungulates in Bialowieza Primeval Forest (Poland and Belarus) in the 19th and 20th centuries. Acta Theriologica 42, 399–451 (1997).
Google Scholar
- 51
Corbett, L. Does dingo predation or buffalo competition regulate feral pig populations in the Australian wet-dry tropics? An experimental study. Wildlife Research 22, 65–74 (1995).
ADS Google Scholar
- 52
Ilse, L. M. & Hellgren, E. C. Resource partitioning in sympatric populations of collared peccaries and feral hogs in southern Texas. Journal of Mammalogy 76, 784–799 (1995).
Google Scholar
- 53
Desbiez, A. L. J., Santos, S. A., Keuroghlian, A. & Bodmer, R. E. Niche partitioning among white-lipped peccaries (Tayassu pecari), collared peccaries (Pecari tajacu), and feral pigs (Sus scrofa). Journal of Mammalogy 90, 119–128 (2009).
Google Scholar
- 54
Gabor, T. M., Hellgren, E. C. & Silvy, N. J. Multi-scale habitat partitioning in sympatric suiforms. The Journal of Wildlife Management 65, 99–110 (2001).
Google Scholar
- 55
Oliveira-Santos, L. G., Dorazio, R. M., Tomas, W. M., Mourao, G. & Fernandez, F. A. No evidence of interference competition among the invasive feral pig and two native peccary species in a Neotropical wetland. Journal of Tropical Ecology 27, 557–561 (2011).
Google Scholar
- 56
Louthan, A. M., Doak, D. F. & Angert, A. L. Where and when do species interactions set range limits? Trends in Ecology & Evolution 30, 780–792 (2015).
Google Scholar
- 57
McClure, M. L. et al. Modeling and mapping the probability of occurrence of invasive wild pigs across the contiguous United States. PLoS ONE 10, e0133771 (2015).
PubMed PubMed Central Google Scholar
- 58
Massei, G. et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Management Science 71, 492–500 (2015).
ADS CAS Google Scholar
- 59
Honda, T. Environmental factors affecting the distribution of the wild boar, sika deer, Asiatic black bear and Japanese macaque in central Japan, with implications for human-wildlife conflict. Mammal Study 34, 107–116 (2009).
Google Scholar
- 60
Morelle, K., Fattebert, J., Mengal, C. & Lejeune, P. Invading or recolonizing? Patterns and drivers of wild boar population expansion into Belgian agroecosystems. Agriculture, Ecosystems & Environment 222, 267–275 (2016).
Google Scholar
- 61
Oliver, W. & Leus, K. Sus scrofa. The IUCN Red List of Threatened Species 2008: e.T41775A10559847. 10.2305/IUCN.UK.2008.RLTS.T41775A10559847.en. (2008).
- 62
Godsoe, W., Franklin, J. & Blanchet, F. G. Effects of biotic interactions on modeled species' distribution can be masked by environmental gradients. Ecology and Evolution 7, 654–664 (2017).
Google Scholar
- 63
Ripple, W. J. et al. Status and ecological effects of the world's largest carnivores. Science 343, 1241484 (2014).
Google Scholar
- 64
Spencer, P. B. & Hampton, J. O. Illegal translocation and genetic structure of feral pigs in Western Australia. Journal of Wildlife Management 69, 377–384 (2005).
Google Scholar
- 65
Skewes, O. & Jaksic, F. M. History of the introduction and present distribution of the european wild boar (Sus scrofa) in Chile. Mastozoología Neotropical 22, 113–124 (2015).
Google Scholar
- 66
Gipson, P. S., Hlavachick, B. & Berger, T. Range expansion by wild hogs across the central United States. Wildlife Society (USA)(1998).
- 67
Brook, R. K. & van Beest, F. M. Feral wild boar distribution and perceptions of risk on the central Canadian prairies. Wildlife Society Bulletin 38, 486–494 (2014).
Google Scholar
- 68
Pedrosa, F., Salerno, R., Padilha, F. V. B. & Galetti, M. Current distribution of invasive feral pigs in Brazil: economic impacts and ecological uncertainty. Natureza & Conservação 13, 84–87 (2015).
Google Scholar
- 69
Lemel, J., Truvé, J. & Söderberg, B. Variation in ranging and activity behaviour of European wild boar Sus scrofa in Sweden. Wildlife Biology 9, 29–36 (2003).
Google Scholar
- 70
Lockwood, J. L., Cassey, P. & Blackburn, T. The role of propagule pressure in explaining species invasions. Trends in Ecology & Evolution 20, 223–228 (2005).
Google Scholar
- 71
Sakai, A. K. et al. The population biology of invasive specie. Annual Review of Ecology and Systematics 32, 305–332 (2001).
Google Scholar
- 72
Warren, R. J., Bahn, V. & Bradford, M. A. The interaction between propagule pressure, habitat suitability and density-dependent reproduction in species invasion. Oikos 121, 874–881 (2012).
Google Scholar
- 73
Syfert, M. M., Smith, M. J. & Coomes, D. A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 8, e55158 (2013).
ADS CAS PubMed PubMed Central Google Scholar
- 74
Barbet-Massin, M., Jiguet, F., Albert, C. H. & Thuiller, W. Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution 3, 327–338 (2012).
Google Scholar
- 75
Kramer-Schadt, S. et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Diversity and Distributions 19, 1366–1379 (2013).
Google Scholar
- 76
Adler, G. H. & Levins, R. The island syndrome in rodent populations. Quarterly Review of Biology 69, 473–490 (1994).
CAS Google Scholar
- 77
Krebs, C. J., Keller, B. L. & Tamarin, R. H. Microtus population biology: demographic changes in fluctuating populations of M. ochrogaster and M. pennsylvanicus in southern Indiana. Ecology 50, 587–607 (1969).
Google Scholar
- 78
Lobo, J. M., Jiménez-Valverde, A. & Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 33, 103–114 (2010).
Google Scholar
- 79
IUCN. The IUCN Red List of Threatened Species. Version 2014.1. http://www.iucnredlist.org. Downloaded on 26 February 2016. (2014).
- 80
Google Earth Engine Team. Google Earth Engine: A planetary-scale geospatial analysis platform. https://earthengine.google.com/. (2016).
- 81
Hawkins, B. A. et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003).
Google Scholar
- 82
Wiens, J. J. et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 13, 1310–1324 (2010).
Google Scholar
- 83
Wiens, J. J. & Graham, C. H. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics 36, 519–539 (2005).
Google Scholar
- 84
Alexander, J. M. & Edwards, P. J. Limits to the niche and range margins of alien species. Oikos 119, 1377–1386 (2010).
Google Scholar
- 85
Fitzpatrick, M. C., Weltzin, J. F., Sanders, N. J. & Dunn, R. R. The biogeography of prediction error: why does the introduced range of the fire ant over-predict its native range? Global Ecology and Biogeography 16, 24–33 (2007).
Google Scholar
- 86
Mau-Crimmins, T. M., Schussman, H. R. & Geiger, E. L. Can the invaded range of a species be predicted sufficiently using only native-range data?: Lehmann lovegrass (Eragrostis lehmanniana) in the southwestern United States. Ecological Modelling 193, 736–746 (2006).
Google Scholar
- 87
Loo, S. E., Nally, R. M. & Lake, P. Forecasting New Zealand mudsnail invasion range: model comparisons using native and invaded ranges. Ecological Applications 17, 181–189 (2007).
Google Scholar
- 88
Broennimann, O. & Guisan, A. Predicting current and future biological invasions: both native and invaded ranges matter. Biology Letters 4, 585–589 (2008).
PubMed PubMed Central Google Scholar
- 89
Beaumont, L. J. et al. Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Diversity and Distributions 15, 409–420 (2009).
Google Scholar
- 90
QGIS Development Team. QGIS 2.14.3 Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. (2016).
- 91
R. R: a language and environment for statistical computing, Version 3.2.3. R Foundation for Statistical Computing. Vienna, Austria. (Development Core Team 2016).
- 92
Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution 1, 103–113 (2010).
Google Scholar
- 93
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical information-theoretic approach. Second Edition., (Springer Verlag, 2002).
- 94
Doherty, P. F., White, G. C. & Burnham, K. P. Comparison of model building and selection strategies. Journal of Ornithology 152, 317–323 (2012).
Google Scholar
- 95
Lukacs, P. M., Burnham, K. P. & Anderson, D. R. Model selection bias and Freedman's paradox. Annals of the Institute of Statistical Mathematics 62, 117–125 (2010).
MathSciNet MATH Google Scholar
- 96
Murtaugh, P. A. Performance of several variable-selection methods applied to real ecological data. Ecology Letters 12, 1061–1068 (2009).
Google Scholar
- 97
Boyce, M. S., Vernier, P. R., Nielsen, S. E. & Schmiegelow, F. K. Evaluating resource selection functions. Ecological Modelling 157, 281–300 (2002).
Google Scholar
- 98
ESRI. ArcGIS Desktop: Version 10.3.1 Environmental Systems Research Institute, Redlands, CA, USA. (2015).
- 99
Geisser, H. & Reyer, H.-u. The influence of food and temperature on population density of wild boar Sus scrofa in the Thurgau (Switzerland). Journal of Zoology 267, 89–96 (2005).
Google Scholar
- 100
Morelle, K. & Lejeune, P. Seasonal variations of wild boar Sus scrofa distribution in agricultural landscapes: a species distribution modelling approach. European Journal of Wildlife Research 61, 45–56 (2015).
Google Scholar
- 101
Sweitzer, R. A. Conservation implications of feral pigs in island and mainland ecosystems, and a case study of feral pig expansion in California. Proceedings of 18th Vertebrate Pest Conference 18 26–34 (1998).
Google Scholar
- 102
Fleming, P. J. et al. In Carnivores of Australia: past, present and future(eds A. S. Glen & C. R. Dickman ) (CSIRO Publishing, 2014).
- 103
Trabucco, A. & Zomer, R. Global soil water balance geospatial database. CGIAR Consortium for Spatial Information. Published online, available from the CGIARCSI GeoPortal at http://cgiar-csi.org (2010).
- 104
Weltzin, J. F. et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 53, 941–952 (2003).
Google Scholar
- 105
Massei, G., Genov, P., Staines, B. & Gorman, M. Mortality of wild boar, Sus scrofa, in a Mediterranean area in relation to sex and age. Journal of Zoology 242, 394–400 (1997).
Google Scholar
- 106
Groves, C. P. Ancestors for the pigs: taxonomy and phylogeny of the genus Sus. 1–96 (Dept. of Prehistory, Australian National University, 1981).
- 107
Bieber, C. & Ruf, T. Population dynamics in wild boar Sus scrofa: ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. Journal of Applied Ecology 42, 1203–1213 (2005).
Google Scholar
Download references
Acknowledgements
This study was funded and supported by the US Department of Agriculture, Animal and Plant Health Inspection Service, Center for Epidemiology and Animal Health, Veterinary Services, Wildlife Services, the National Wildlife Research Center, the National Feral Swine Damage Management Program, Colorado State University, and Conservation Science Partners. We appreciate the distribution data for wild pigs in Canada provided by R. Kost and R. Brook, synthesis of wild pig distribution in Africa and South America by C. Larson, and the dingo distribution data in Australia provided by P. Fleming. P. DiSalvo and M. Foley assisted with acquiring literature on density estimates. M. McClure assisted with cross validation of model results. We thank three anonymous reviewers, S. Sweeney and B. Dickson for providing thoughtful feedback that improved earlier versions of this paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and Permissions
About this article
Cite this article
Lewis, J., Farnsworth, M., Burdett, C. et al. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci Rep 7, 44152 (2017). https://doi.org/10.1038/srep44152
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/srep44152
Further reading
-
Epidemiological analysis of classical swine fever in wild boars in Japan
BMC Veterinary Research (2021)
-
Wetland hydroperiod predicts community structure, but not the magnitude of cross-community congruence
Scientific Reports (2021)
-
Behavioral state resource selection in invasive wild pigs in the Southeastern United States
Scientific Reports (2021)
-
The global impact of wild pigs (Sus scrofa) on terrestrial biodiversity
Scientific Reports (2021)
-
Deterring non-target birds from toxic bait sites for wild pigs
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Chapter 52 Reading Guide Biotic and Abiotic Factors
Source: https://www.nature.com/articles/srep44152
0 Response to "Chapter 52 Reading Guide Biotic and Abiotic Factors"
Post a Comment